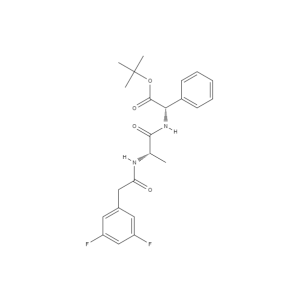
DAPT
description
Additional Information
|
Applications:
|
FA
|
|
Synonyms:
|
gamma-Secretase Inhibitor IX, GSI IX, N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine tert-butyl ester
|
|
Formulation:
|
Crystalline solid
|
|
Chemical Name:
|
tert-butyl (2S)-2-[[(2S)-2-[[2-(3,5-difluorophenyl)acetyl]amino]propanoyl]amino]-2-phenylacetate
|
|
Molecular Formula:
|
C23H26F2N2O4 |
|
Molecular Weight: |
432.5
|
|
CAS Number
|
208255-80-5
|
|
Purity:
|
≥95%
|
|
Storage Conditions:
|
Product should be kept at -20°C.
|
|
References:
|
El Mouedden, M., Vandermeeren, M., Meert, T., & Mercken, M. (2005). Reduction of Abeta levels in the Sprague Dawley rat after oral administration of the functional gamma-secretase inhibitor, DAPT: a novel non-transgenic model for Abeta production inhibitors. Current pharmaceutical design, 12(6), 671-676. Yagishita, S., Morishima-Kawashima, M., Tanimura, Y., Ishiura, S., & Ihara, Y. (2006). DAPT-induced intracellular accumulations of longer amyloid β-proteins: further implications for the mechanism of intramembrane cleavage by γ-secretase. Biochemistry, 45(12), 3952-3960. Crawford, T. Q., & Roelink, H. (2007). The Notch response inhibitor DAPT enhances neuronal differentiation in embryonic stem cell‐derived embryoid bodies independently of sonic hedgehog signaling. Developmental Dynamics,236(3), 886-892. Grottkau, B. E., Friedrich, C. C., Jing, W., & Wu, Y. (2009). DAPT enhances the apoptosis of human tongue carcinoma cells. International journal of oral science, 1(2), 81-89. |